Superconductors
States of matter that let current flow indefinitely—a cool feat in more ways than one.
The electrical wiring behind your walls may seem passive, but behind that calm façade is a bustling, microscopic mess. Electrons, the
Wouldn’t it be great if electrons could just cruise through a wire without that pesky energy loss? Why, yes. That would be super.
In fact, that’s exactly what happens inside of certain materials called superconductors: Electrons flow without any resistance. This means that superconductors don’t really heat up, so they can carry huge amounts of current—levels that would ordinarily melt a metal.

Under the right circumstances, that current can keep going and going and going. In one experiment, scientists observed current circulating inside a superconductor for more than 20 years. It’s like stirring your tea and watching it swirl around forever without bothering to slow down as it drags against the sides of your mug.
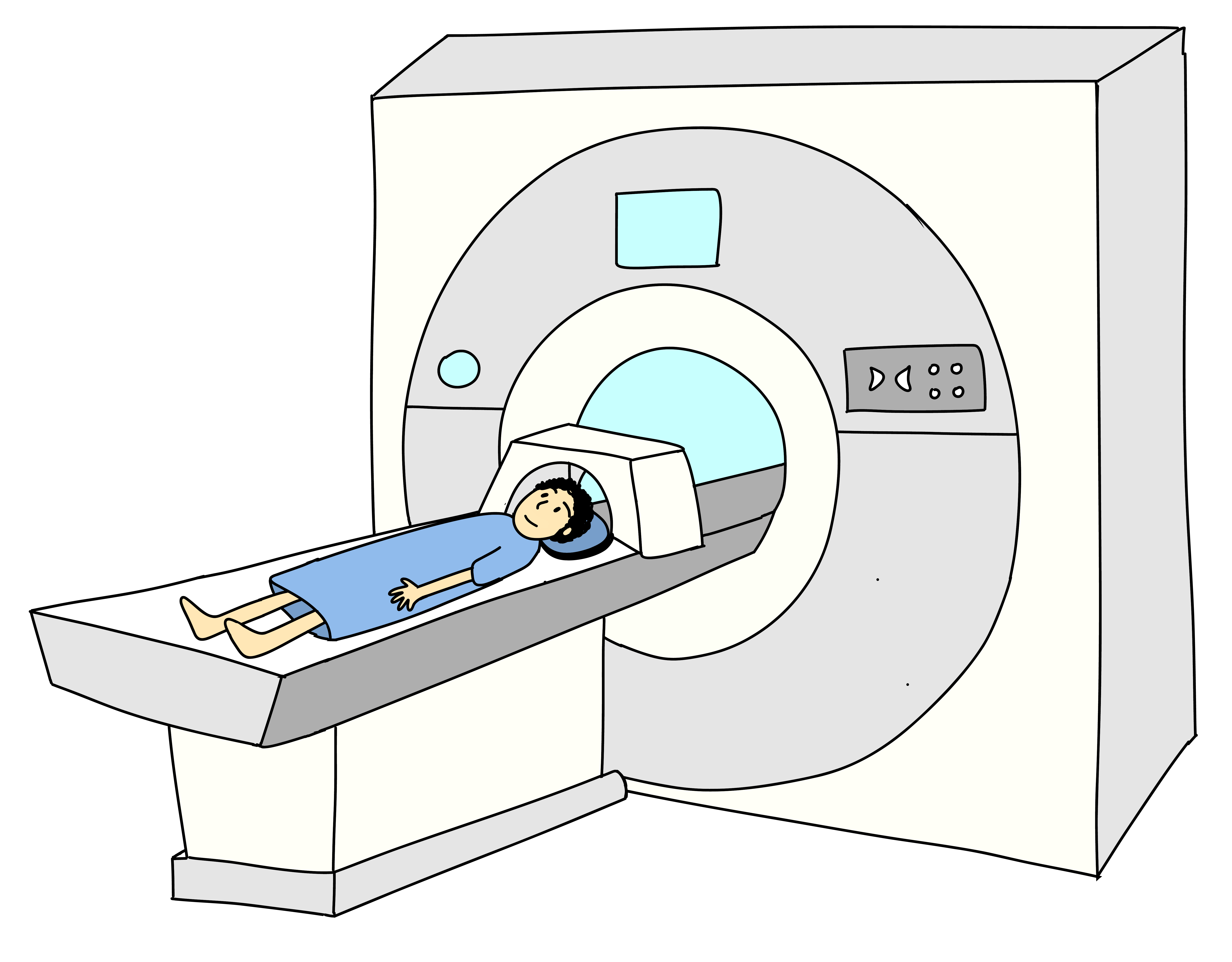
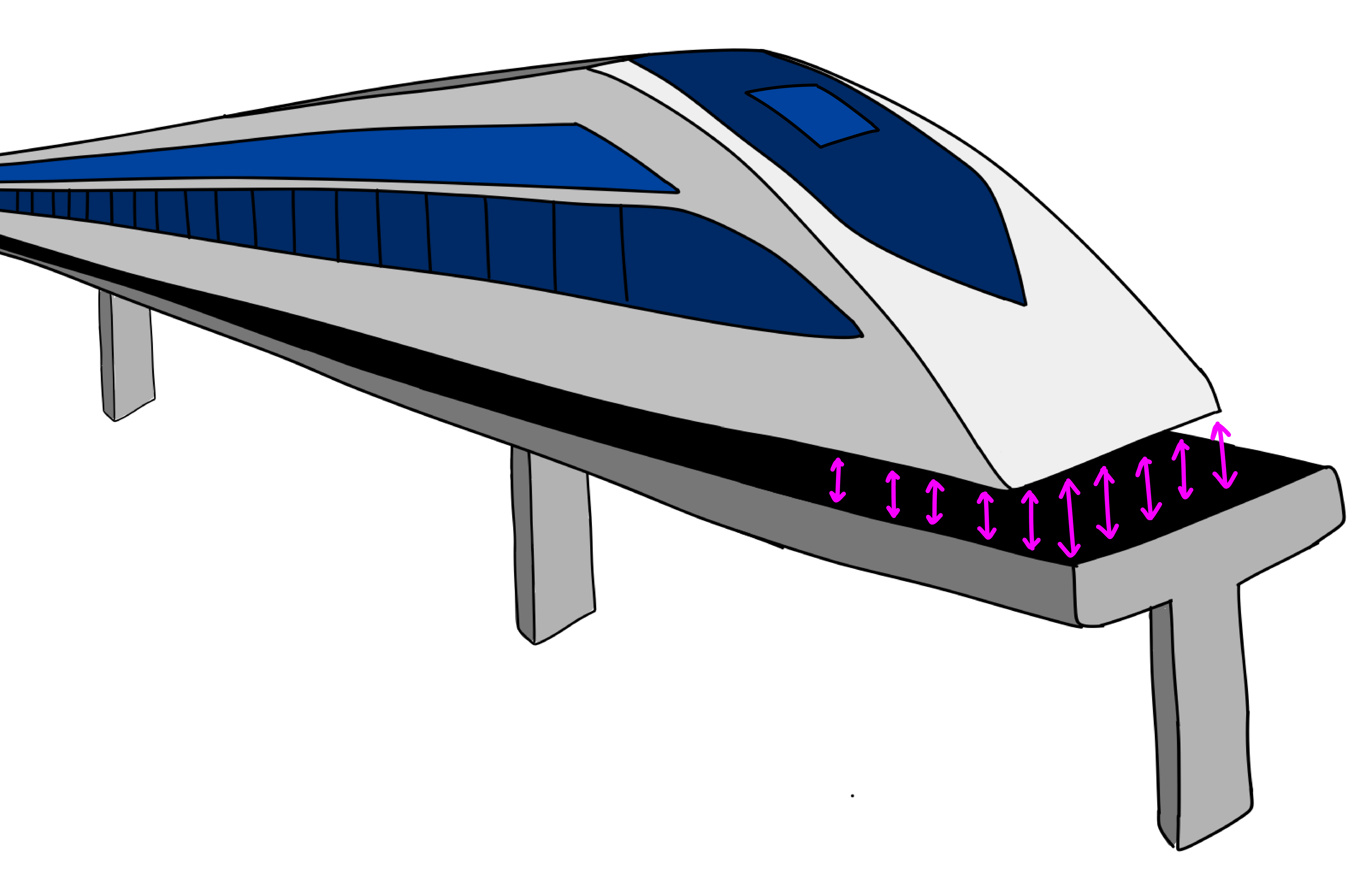
All this makes superconductors superb choices for current-hungry applications like MRI machines, enormous physics experiments, and even experimental trains. There are also small quantum computers that store and process quantum information in circuits made from superconductors.
But before you rip out all the copper wires behind your walls and replace them with superconductors, take note: Superconductors aren’t quite ready for home use. First, many are very brittle, which is not great for making wires. Also, most superconductors only work when they are super cold. We’re talking a few hundred degrees below zero (Celsius or Fahrenheit). Any balmier and the materials lose those superpowers, turning into comparatively boring old conductors or insulators.
For decades, scientists have been on a quest to find superconductors that are both less brittle and more tolerant of room temperature. The search goes on, and we now know about many more materials that can be “super.” But the perfect unicorn superconductor remains elusive.
A Quantum Origin Story
At the heart of every superconductor is a

This pairing is kind of unusual. For one, electrons normally repel each other because they have the same electric charge. So at low temperatures, something (like those vibrations) has to emerge and overwhelm the repulsion.
But the pairing is strange for another, almost alchemical reason. On their own, electrons are spin-1/2 particles, which means they can’t pile into the
Once many electrons pair off like this, they can inhabit the lowest-energy quantum state. And when they've collected there, they are shielded from disruptive collisions with the surrounding atoms. That's because the collisions can't supply enough energy to break the pairs apart, which makes the current in a superconductor very stable.
Unfortunately for most of the superconductors discovered by scientists,
Beyond the cool conducting effects, superconductors are pretty super for other reasons, too. One is the Meissner effect and another is the closely related phenomenon of flux pinning. The Meissner effect (shown in the video at the top of this entry) results in a superconductor becoming allergic to magnetic fields: As materials turn into superconductors, they expel all magnetic fields, redirecting them over and around the surface. This makes the superconductor itself act a bit like a magnet, since it repels magnetic fields in its vicinity. The result is that magnetic objects can levitate above a superconductor and vice versa.
Other superconductors can exhibit flux pinning. Instead of only repelling fields, these superconductors will allow some of the field to pierce through. Bits of the field called flux get pinned inside the material, which leads to some of the more breathtaking images of "quantum levitation." In this effect, the superconductor resists any changes to its magnetic surroundings, so if you manage to get the superconductor hovering above or below a magnet, chances are it will hang out there until it warms up.


